Why Pool pH Drifts — and How to Stabilise It Properly
If you’re constantly adding acid or buffer to your pool, you’re not alone.
One of the most common frustrations pool owners and service technicians face is pH that won’t stay put — even when test results look “correct” one day and wrong the next.
The real cause is usually misunderstood.
This article explains:
- Why pool pH naturally drifts
- The hidden role of total alkalinity
- Why chasing pH alone never works
- How to stabilise pool water properly — with confidence
What pH Actually Measures (and Why It’s Unstable)
pH measures the concentration of hydrogen ions (H⁺) in water.
In pools, pH is affected by:
- Aeration (waterfalls, spillways, jets)
- Rain and top-up water
- Chlorine dosing
- Swimmer load
- Temperature changes
- Carbon dioxide (CO₂) loss to the air
Even when no chemicals are added, pH will still drift — usually upwards.
This is normal chemistry, not a fault.
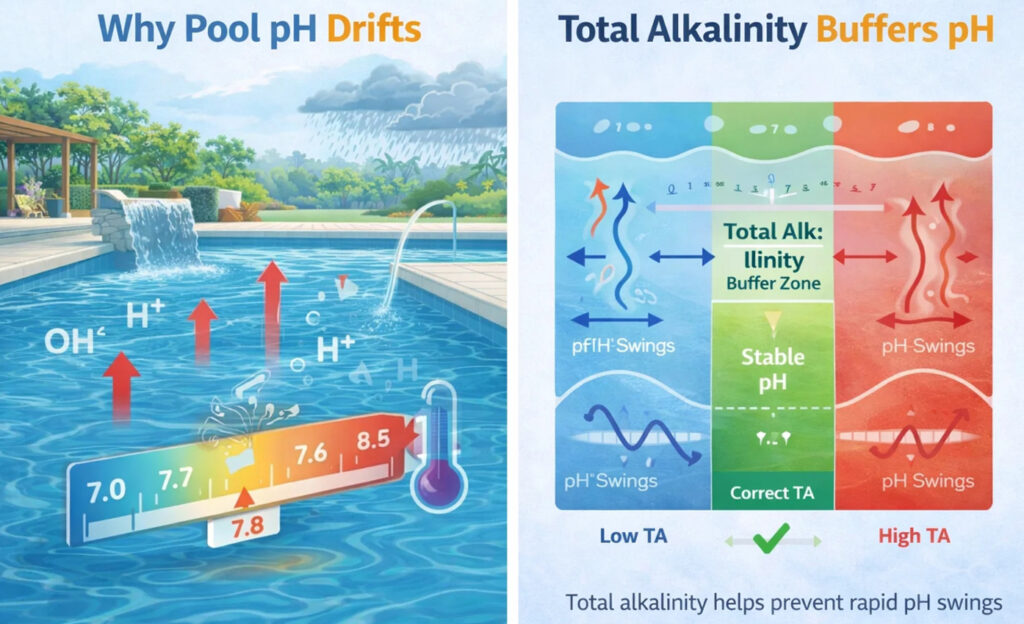
Why Pool pH Almost Always Drifts Upwards
The most common cause of pH rise is CO₂ off-gassing.
When water is aerated:
- CO₂ escapes into the air
- Carbonic acid decreases
- pH rises
This happens faster when:
- Water features are running
- Pumps run longer
- Pools are heated
- Wind and splashing increase
Adding acid lowers pH temporarily — but doesn’t fix the cause.
The Missing Piece: Total Alkalinity
Total Alkalinity (TA) is NOT pH
Total alkalinity measures the water’s buffering capacity — its ability to resist rapid pH change.
Think of it like suspension in a car:
- pH is the position of the car
- Alkalinity is the shock absorber
Without proper alkalinity, pH will swing wildly.
What Happens When Alkalinity Is Wrong
Low Total Alkalinity
- pH becomes unstable
- Small additions cause large swings
- Acid demand increases
- Corrosion risk rises
High Total Alkalinity
- pH constantly drifts upward
- Acid is consumed rapidly
- Scale formation increases
- Chlorine becomes less effective
Both extremes cause problems — but for different reasons.
The “Chasing pH” Trap
Many pool owners fall into this cycle:
- pH reads high → add acid
- pH drops quickly
- pH rises again within days
- More acid is added
- Water becomes chemically unstable
The problem isn’t the acid.
The problem is unbalanced alkalinity.
The Correct Order: Alkalinity First, pH Second
To stabilise pH properly:
- Measure total alkalinity
- Adjust TA into the correct range
(typically 80–120 ppm for most pools — confirm for your system) - Allow water to circulate
- Adjust pH only after TA is correct
Once alkalinity is stable, pH:
- Drifts slower
- Requires fewer corrections
- Becomes predictable
Why Digital Measurement Matters
Test strips often:
- Mask gradual drift
- Miss instability
- Encourage over-correction
A digital pH meter allows you to:
- See trends, not guesses
- Detect slow drift early
- Verify stabilisation properly
For reliable results:
- Calibrate regularly
- Store the probe correctly
- Use known buffer solutions
Stable Water = Confident Decisions
Stable pool water isn’t about adding more chemicals.
It’s about understanding the chemistry and measuring it accurately.
When alkalinity is correct:
- pH stops “wandering”
- Chemical use decreases
- Water becomes easier to manage
- Equipment lasts longer
That’s what measurement with confidence looks like.